Tactical Rehabilitation offers a variety of ultrasound devices and bone growth stimulators.

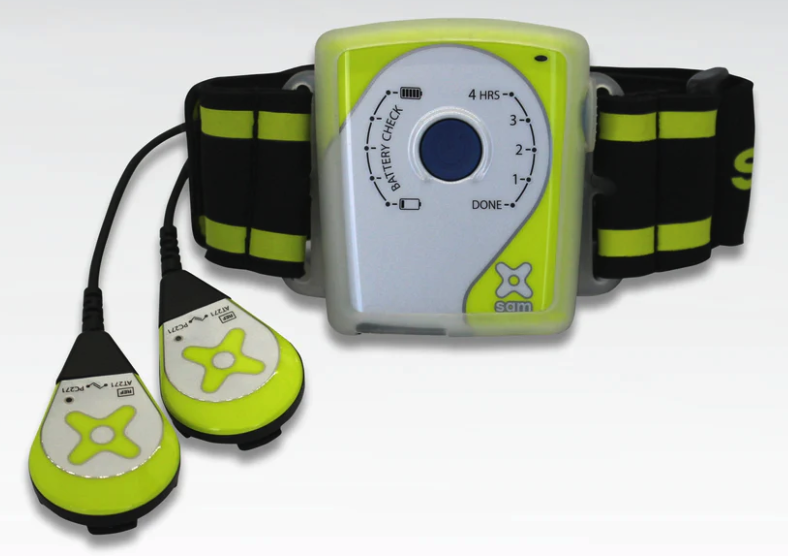
Sam® Pro 2.0
Sam® Pro is a sustained acoustic medicine product offering single rapid charge, touch control, enhanced coupling patches, and rugged housing. The device is designed for patients with arthritis and chronic injuries. This product is easy to use, safe, and effective. Sam® Pro is approved by the US FDA. It is clinically proven to reduce pain and improve function. It has helped 24 million US Veterans and active duty service members.
Click Here for Ordering Info
Ordering Info for DME Consult:
This is an order for a Sam Pro 2.0 E1399-6 as listed from Tactical Rehabilitation INC (Fed Tax ID 46-2082171) including one unit E1399-1 and 5 packs of patches E1399-5.
Information to be dictated in each patient’s medical notes:
I am prescribing the SAM® 2.0 wearable-active multi-hour Low Intensity ultrasound device (up to 4-hours per day) to reduce pain and accelerate musculoskeletal healing for this patient. This device is prescribed to control pain and to reduce/eliminate the need for prescription pain medication including opioids. SAM® 2.0 is not a traditional Therapeutic Ultrasound which is only used for 5-8 minutes in a clinic which does not accelerate healing or reduce pain. SAM® 2.0 should be used for up to 4-hours per day for up to 60-days and is clinically proven
to accelerate collagen lay down, increase oxygenated hemoglobin in the muscles, accelerate angiogenesis effect for capillary development, and to increase local blood flow. The SAM® device may be used at home or at work.
Commonly qualified Dx used for referral:
Diagnosis similar/related, but not limited to, most/all t endonitis, bursitis, muscle tears, biceps impingement, SI dysfunction, facet syndrome, and osteoarthritis of all joints, etc.
NOT COVERED:
Generic Dx codes only showing symptom, i.e. General & Low Back Pain. Dx must show specific, underlying condition.



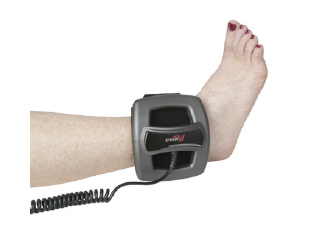
CMF OL1000™ Bone Growth Stimulators
CMF OL1000™ Bone Growth Stimulators are portable, battery-powered medical devices indicated for use in the noninvasive treatment of an established nonunion fracture acquired secondary to trauma, excluding all vertebrae and flat bones. The device was designed with the following features: Lightweight and comfortable, easy-to-use & noninvasive. Requires simple, one-button operation. Device is worn for 30 minutes per day, Can be used with internal or external fixation or over a cast. The CMF signal continuously operates within frequencies optimal to heal bone. This potent signal allows for a single 30-minute per day treatment and is specifically targeted to the osteoblast cell. This signal targets the endogenous production of IGF-II , a key growth factor in the formation of bone. GF-II protein is recruited through calcium ion channels and receptors on the cell membrane. The resulting increase in IGF-II growth factor helps promote cell division. CMF was specifically designed for a once daily treatment to best mimic cell replication. As the cells continue to divide and regular treatment progresses, the body is able to heal.
Click Here for Ordering Info
Ordering Info for DME Consult:
This is an order for a DonJoy CMF OL1000 E0747-1 Bone Growth Stimulator as listed from Tactical Rehabilitation INC (Fed Tax ID 46-2082171)
*Needs CMN for billing (Tricare may request in order to secure auth).
Indications
- Diagnosis for either the specific fracture site or the non union for the specific fracture site.
- Fractures greater than 14 days showing nonunion and no progressive signs of healing
- Fracture gap required
- Follows FDA Guidelines (No joint fusion coverage)
Contraindications:
- Use of this device is contraindicated in individuals having a synovial pseudarthrosis.
- Demand-type pacemaker or implantable cardiovertor defibrillator (ICD) operation may be adversely affected by exposure to magnetic fields. Physicians should not prescribe CMF™ OL1000™ for applications that may place the treatment transducers in close proximity to the pacemaker.




CMF Spinalogic Bone Growth Stimulator
CMF SpinaLogic is a portable, battery-powered, micro-controlled, noninvasive bone growth stimulator indicated as an adjunct electromagnetic treatment to primary lumbar spinal fusion surgery for one or two levels. Designed to be a lightweight device with cushion strap designed for maximum patient comfort. Can be applied over a cast, brace, or clothing. DJO’s more advanced technology to support patient use and one button technology for ease of use.
Indications
- Lumbar only coverage
- Adjunct to Primary lumbar spinal fusion – single or multi-level L1,L2,L3,L4,L5
- Single level fusion w/ Risk factors
- Current Tobacco use
- Diabetes
- Obesity
- Steroid use
- Grade 2 or higher spondy
- Renal disease
- Severe Anemia
- Follows FDA Guidelines
*After surgery with no specific time frame identified
(No revision fusion, No failed spinal fusion coverage)
Click Here for Ordering Info
Ordering Info for DME Consult:
This is an order for a DonJoy CMF Spinalogic E0748-1 Bone Growth Stimulator as listed from Tactical Rehabilitation INC (Fed Tax ID 46-2082171)
*Needs CMN for billing (Tricare may request in order to secure auth).
**Criteria for Coverage: non-union fracture with no progression of healing
Contraindications:
- Demand-type pacemaker and implantable cardiovertor defibrillator (ICD) operation may be adversely affected by exposure to combined static and dynamic magnetic fields. Physicians should not prescribe CMF™ SPINALOGIC® for patients with such devices.
- The safety and effectiveness of CMF™SPINALOGIC® in pregnant women have not been studied, and the effects of the device on the mother or the developing fetus are unknown. Thus, this device should not be used in pregnant women. If a woman becomes pregnant during treatment with CMF™ SPINALOGIC®, treatment should be discontinued immediately.
